
Key Findings
- Most white label cosmetics cannot use the supplier's CPSR—you need your own as the brand owner and Responsible Person
- Under EU Regulation 1223/2009, each brand placing products on the market must have a valid CPSR in their name
- Pre-assessed white label products may allow CPSR transfer, but your Responsible Person must validate and assume liability
- CPSR preparation takes 6-8 weeks and costs between €500-2,000 per product, depending on complexity
The Direct Answer: When You Need a New CPSR
Here's what most white label cosmetics brands don't expect: you almost always need a new Cosmetic Product Safety Report, even when using your supplier's formulation. While your supplier's CPSR proves their product is safe, you—as the brand owner placing the product on the EU market—become the Responsible Person. This legal role requires a CPSR in your company's name.
The only exception exists when working with pre-assessed white label products where the supplier has specifically structured their CPSR to allow transfer to multiple brand owners. Even then, your designated Responsible Person must review, validate, and formally accept that safety assessment before you can legally sell in the EU.
The requirement stems from a fundamental principle in EU cosmetics regulation: the entity whose name appears on the product label bears full legal responsibility for safety. Your supplier's CPSR protects them—not you. Regulatory authorities will hold you accountable if anything goes wrong.
What Is a CPSR and Why It Matters for White Label Brands
A Cosmetic Product Safety Report is the cornerstone of EU cosmetics compliance. This comprehensive document demonstrates that your product is safe for human health when used under normal or reasonably foreseeable conditions. Every cosmetic product sold in the EU must have a valid CPSR as part of its Product Information File.
The Two-Part Structure of a CPSR
Under Regulation EC 1223/2009, a compliant CPSR consists of two mandatory parts:
Part A: Product Safety Information contains the scientific data supporting safety. This includes complete formulation details with INCI names and concentration ranges, physicochemical specifications, microbiological quality standards, purity of ingredients, impurity testing results, packaging specifications and compatibility studies, stability data demonstrating shelf life, and toxicological profiles for each ingredient.
Part B: Cosmetic Product Safety Assessment is where the qualified safety assessor evaluates the data. This section includes exposure calculations based on intended use, toxicological risk assessment for each ingredient, evaluation of ingredient interactions, assessment of restricted substances compliance, conclusion on product safety, labeling and usage instructions review, and any warnings or precautions required.

Who Can Sign a CPSR?
Not just anyone can validate product safety. EU regulations require the safety assessor to possess a diploma or equivalent qualification in pharmacy, toxicology, medicine, or a similar discipline. This professional must be able to demonstrate their qualifications to competent authorities upon request.
When you commission a new CPSR for your white label product, you're paying for this qualified expert's time to review all safety data, perform toxicological calculations, and stake their professional reputation on their safety conclusion. This is why CPSRs cannot simply be copied—each assessment represents a legal liability that the assessor assumes.
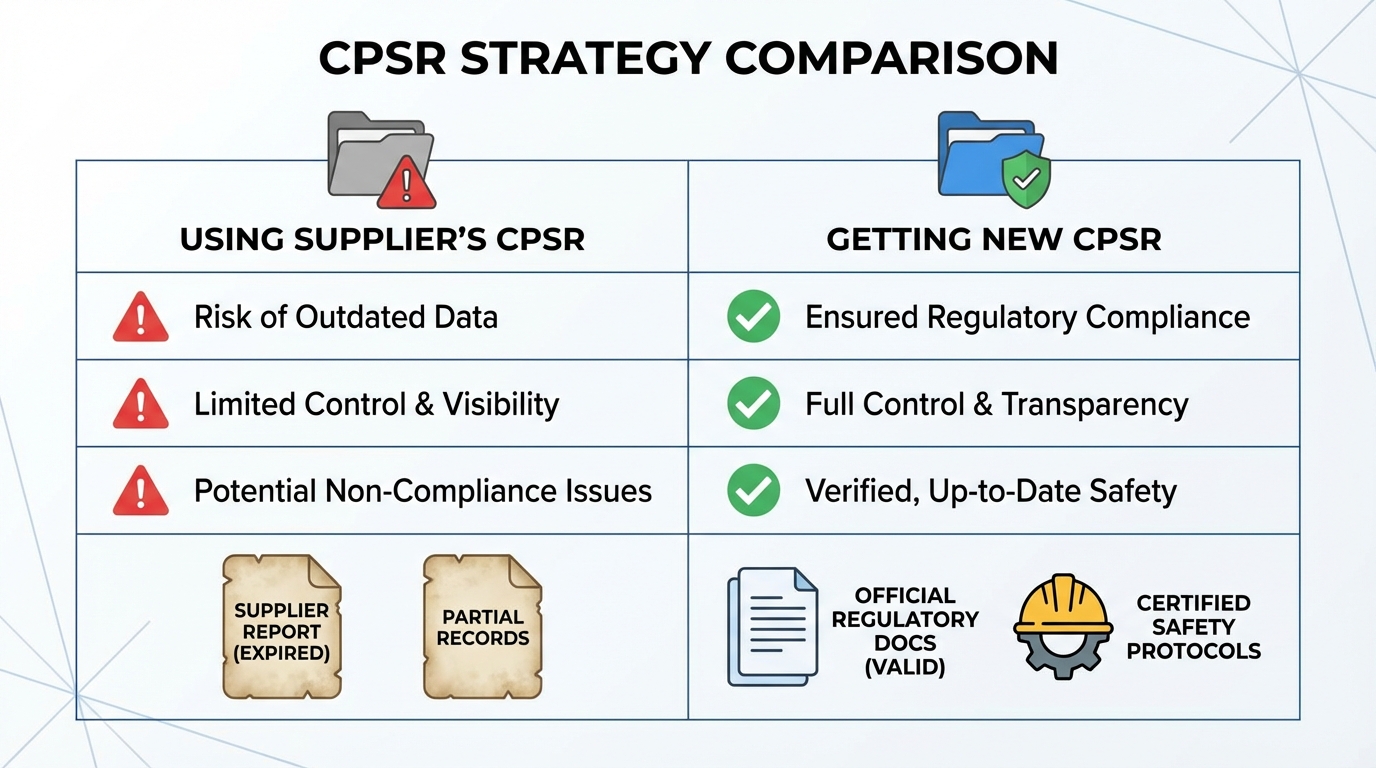
Can You Use Your Supplier's CPSR? The Nuanced Reality
The straightforward answer is that most of the time, no—you cannot directly use your supplier's CPSR. But the complete picture is more nuanced, and understanding these distinctions can save you thousands of euros.
Standard White Label Scenarios
In typical white label arrangements, your supplier manufactures products and holds their own CPSR. Their documentation lists their company as the Responsible Person. When you purchase these products to rebrand and sell, you become the new Responsible Person for your market.
Here's the critical issue: the CPSR is legally tied to the Responsible Person named in the document. Your supplier's safety assessor evaluated the product's safety under your supplier's quality systems, manufacturing controls, and regulatory oversight. That assessment doesn't automatically transfer to you.
"A common misconception is that buying a white label product means buying its compliance documentation. In reality, you're buying a formulation and manufacturing service. The regulatory responsibility transfers to you, but the compliance documentation often cannot."
Pre-Assessed White Label Products: The Exception
Some sophisticated white label suppliers have addressed this challenge by creating what industry professionals call "pre-assessed" or "master file" CPSRs. These documents are specifically structured to support multiple brand owners.
In these arrangements, the supplier's safety assessor prepares a comprehensive CPSR that can be licensed or transferred to multiple clients. Your Responsible Person reviews this master assessment, confirms all information applies to your specific scenario, and formally adopts it for your brand.
Even with pre-assessed products, you still need an EU-based Responsible Person who reviews and validates the assessment. This person must confirm that the formulation, manufacturing standards, and safety conclusions align with your product as you intend to market it. You cannot skip this step.
What Documentation Can Be Shared?
While the CPSR itself typically cannot be directly reused, certain underlying documentation can support your new CPSR preparation:
- Complete formulation data with INCI names and concentration ranges
- Stability testing results demonstrating shelf life
- Microbiological testing and challenge test data
- GMP certificates for the manufacturing facility
- Ingredient safety data sheets and toxicological profiles
- Packaging compatibility and migration testing
When your supplier provides this documentation, your safety assessor can prepare your CPSR more efficiently. The assessor still must independently review everything and issue their own safety conclusion in your name, but having comprehensive data reduces both time and cost.
The Responsible Person Requirement: Your Legal Obligation
Understanding the Responsible Person role is fundamental to grasping why white label cosmetics need their own CPSR. This isn't a bureaucratic formality—it's the legal entity that competent authorities will hold accountable for product safety.
What Is a Responsible Person?
Article 4 of Regulation EC 1223/2009 requires every cosmetic product placed on the EU market to have a designated Responsible Person. This must be a natural or legal person established within the European Union who ensures the product complies with all regulatory requirements.
The Responsible Person cannot be outside the EU. If you're a brand based in the United States, United Kingdom (post-Brexit), Asia, or anywhere outside the European Economic Area, you must appoint an EU-based Responsible Person. This can be your own EU subsidiary or an external regulatory affairs company that provides Responsible Person services.
Key Responsibilities That Require Your Own CPSR
The Responsible Person must ensure several critical compliance elements, each of which ties directly to the CPSR:
Maintain a Product Information File: Your Responsible Person must keep a complete PIF that includes the CPSR, GMP documentation, and proof of claimed effects. This file must be readily available to competent authorities for at least 10 years after the last batch was placed on the market. Your supplier's PIF doesn't satisfy this requirement—you need your own.
Notify the Product via CPNP: Before placing your product on the market, your Responsible Person must submit a notification through the Cosmetic Product Notification Portal. This notification explicitly links your company, your product, and your CPSR. You cannot use your supplier's CPNP notification.
Ensure Label Compliance: Your product label must display your Responsible Person's name and address. Competent authorities use this information to contact the liable party if safety concerns arise. The label ties directly to your CPSR—not your supplier's.
Implement Safety Monitoring: If adverse reactions occur or new safety information emerges, your Responsible Person must take corrective action. This includes updating your CPSR, modifying product formulation or labeling, or withdrawing the product from the market. You cannot rely on your supplier to monitor and respond to issues affecting your brand.
Using External Responsible Person Services
If you don't have an EU presence, you'll need to contract with a Responsible Person service provider. These regulatory affairs companies offer RP services as part of a broader compliance package. Typical costs range from €500-1,500 per product annually, covering CPSR preparation or validation, PIF maintenance, CPNP notification, ongoing regulatory monitoring, and liaison with competent authorities if issues arise.
When evaluating RP service providers, verify they employ qualified safety assessors with appropriate credentials. Ask for references from other white label brands in your product category. Confirm they maintain professional indemnity insurance covering their RP services.
When White Label Products Require a New CPSR: Specific Scenarios
Let's examine the specific circumstances that trigger the need for a new CPSR when working with white label cosmetics. Understanding these scenarios helps you plan accurately and avoid compliance surprises.
Any Formulation Modifications
If you make any changes to the formulation—even minor adjustments—you need a new or amended CPSR. This includes changing preservative systems, adjusting fragrance concentrations, modifying active ingredient levels, substituting raw material suppliers (which may affect impurity profiles), or altering pH or other physicochemical properties.
Even changes you consider cosmetic, like adjusting colorants or fragrances, require toxicological review. The safety assessor must evaluate whether the modification affects the product's overall safety profile.
Rebranding Under Your Company Name
When you purchase white label products and apply your own branding, you trigger the CPSR requirement. Your company becomes the entity placing the product on the market. The regulatory framework treats this as a new market placement requiring full compliance documentation in your name.
This applies even if the physical product is identical to what your supplier sells under their own brand. The legal responsibility shifts, so the compliance documentation must shift accordingly.
Different Target Markets or Claims
If you intend to market the product differently than your supplier—making different claims, targeting different consumer groups, or suggesting different usage patterns—you need a new CPSR that reflects your marketing approach.
For example, if your supplier markets a face cream for normal skin but you want to market it as an anti-aging treatment, the safety assessor must evaluate whether the formulation supports that claim and whether different warnings or usage instructions are required.
Packaging or Container Modifications
Changing product packaging may require CPSR updates. If you switch from the supplier's original packaging to your own containers, the safety assessor must evaluate packaging compatibility. Different materials may interact with the formulation, affecting stability or introducing contaminants through migration.
Common scenarios include switching from plastic to glass containers, changing pump dispensers or applicators, altering container size which affects preservation systems, or using different barrier materials for sensitive ingredients.
Supplier Cannot Provide Transfer Documentation
Many white label suppliers, particularly smaller manufacturers or those outside the EU, simply don't have the infrastructure to provide transferable CPSRs. If your supplier cannot supply a pre-assessed master file or addendum naming you as an authorized user, you must commission your own CPSR.
This is particularly common when working with manufacturers in Asia, the United States, or other non-EU regions who may not fully understand EU compliance requirements. Don't assume your supplier's "safety certificates" or "test reports" substitute for a proper CPSR.
| Scenario | Can Use Supplier's CPSR? | What You Need |
|---|---|---|
| Exact product, supplier offers pre-assessed CPSR | Yes (with validation) | RP review, CPSR addendum, your own PIF |
| Exact product, standard white label | No | New CPSR in your name |
| Modified formulation | No | New CPSR with updated Part B |
| Different packaging | Maybe | CPSR amendment after compatibility testing |
| Different claims or usage | No | New CPSR with appropriate exposure assessment |
| Non-EU supplier | Unlikely | New CPSR plus EU Responsible Person |
How to Obtain a New CPSR for White Label Products
Once you've determined you need a new CPSR, the process follows a structured workflow. Understanding each step helps you gather documentation efficiently and avoid delays.
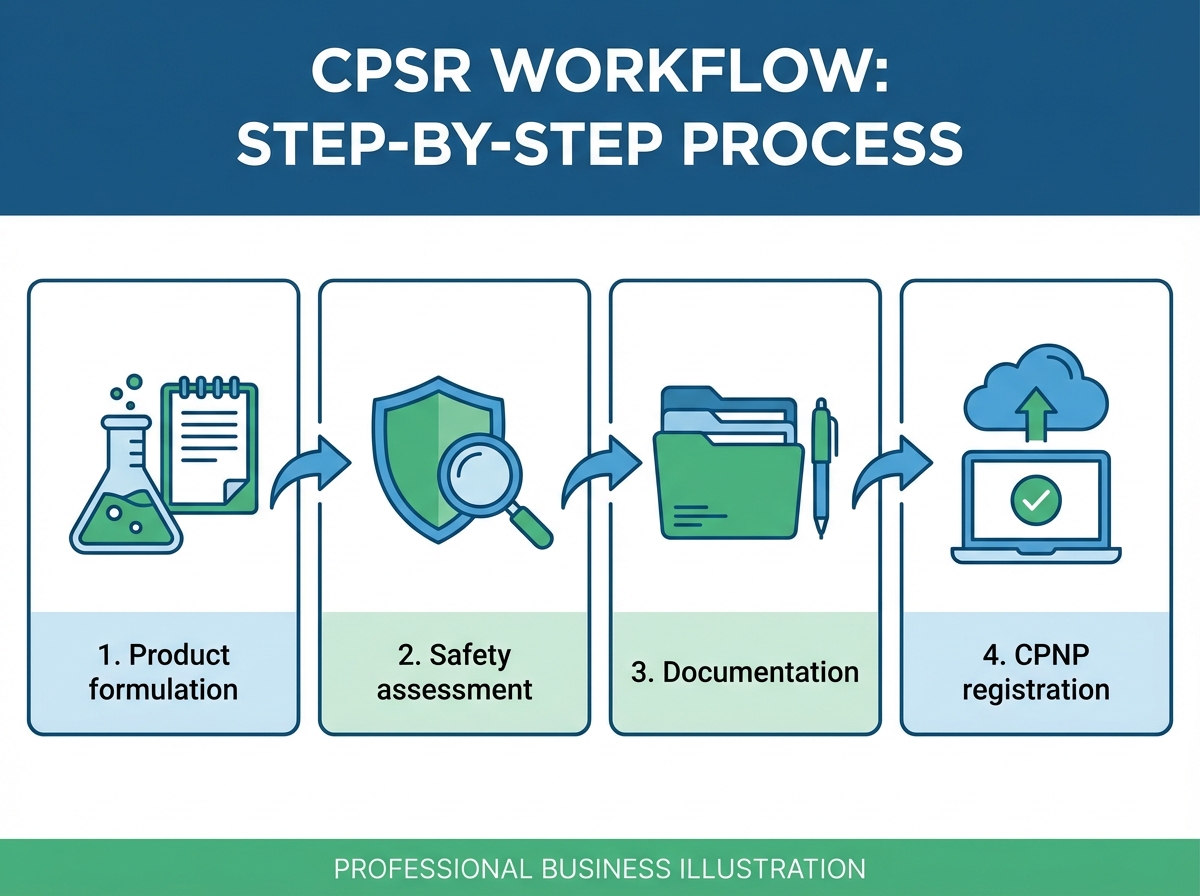
Step 1: Gather Complete Formulation Information
Start by obtaining comprehensive formulation data from your supplier. You need the complete formula with INCI names for every ingredient, exact concentration ranges (percentages by weight), function of each ingredient in the formulation, CAS numbers and EC numbers for identification, and specifications for raw materials including purity and potential impurities.
Many suppliers hesitate to disclose complete formulation details, viewing this as proprietary information. Explain that you need this data for legal compliance—not to reverse-engineer their product. Emphasize that your safety assessor will sign confidentiality agreements.
If your supplier refuses to provide formulation details, you face two options: find a different supplier who understands EU compliance requirements, or commission laboratory analysis to reverse-engineer the formulation (expensive and time-consuming).
Step 2: Collect Supporting Test Data and Certifications
Request all supporting documentation that demonstrates product safety and quality:
- Stability testing data: Results showing the product remains safe and effective throughout its claimed shelf life under various storage conditions
- Challenge testing: Microbiological studies proving the preservation system effectively prevents microbial contamination
- Safety data sheets: For each ingredient, especially any that are classified as hazardous or restricted
- GMP certificates: Documentation that the manufacturing facility complies with ISO 22716 or equivalent good manufacturing practices
- Packaging compatibility: Testing showing no adverse interactions between the formula and container materials
Not all suppliers will have conducted every test. If critical data is missing, you may need to commission additional testing before your safety assessor can complete the CPSR. Stability testing and microbiological testing are particularly common gaps.
Step 3: Select a Qualified Safety Assessor
Choose a cosmetic safety assessor or regulatory affairs consultancy to prepare your CPSR. Look for professionals or firms with appropriate scientific credentials (pharmacy, toxicology, dermatology), proven experience with your product category, understanding of white label business models, and the ability to serve as your Responsible Person if needed.
When evaluating assessors, ask about their typical turnaround times, what documentation they require from you, whether they charge per product or offer package pricing, and what ongoing support they provide if formulations or regulations change.
Step 4: CPSR Preparation and Review
Once you've engaged an assessor and provided all documentation, they begin CPSR preparation. The process typically includes ingredient-by-ingredient toxicological review, exposure calculation based on your intended use, evaluation against restricted substances lists in Annexes II through VI of the Cosmetics Regulation, assessment of labeling requirements and warnings, and conclusion on overall product safety.
The assessor will likely have follow-up questions about formulation details, intended use patterns, target consumer groups, and any claims you plan to make. Respond promptly—delays in providing information extend the timeline.
For straightforward products like simple moisturizers or shampoos with well-established ingredients, CPSR preparation typically takes 4-6 weeks. Complex products with novel ingredients, multiple actives, or challenging formulations may require 8-12 weeks.
Step 5: CPSR Approval and Documentation Management
When your safety assessor completes the CPSR, they'll issue the signed document with their professional qualifications clearly stated. This CPSR becomes part of your Product Information File, which must be maintained for 10 years after the last batch enters the market.
Store your PIF securely but accessibly. Competent authorities can request it at any time, and you must produce it quickly. Many brands maintain digital copies in secure cloud storage with backup physical copies.
Essential Documentation You'll Need from Your White Label Supplier
Success in obtaining a CPSR efficiently depends on having complete documentation from your supplier. Here's your detailed checklist of what to request, organized by priority.
Critical Documents (Cannot Proceed Without These)
Complete Formulation Specification: Full ingredient list with INCI names, concentration ranges for each ingredient (minimum and maximum percentages), manufacturing instructions and order of addition, and pH range and other key physicochemical parameters.
Manufacturing Documentation: Name and address of the manufacturing facility, GMP certificate (ISO 22716 or equivalent), manufacturing process description, and quality control procedures and specifications.
Safety Testing Results: Stability testing under relevant conditions (ambient, elevated temperature, light exposure), microbiological challenge testing or preservative efficacy testing, and compatibility testing with packaging materials.
Ingredient Safety Data: Safety Data Sheets for all ingredients, certificates of analysis from raw material suppliers, and information on impurities or contaminants in ingredients.
Highly Recommended Documents
While these aren't absolute requirements, having them significantly streamlines CPSR preparation:
- Heavy metals testing results
- Batch analysis reports from actual production
- Analytical method validation for quality control tests
- Allergen analysis if fragrances are present
- Efficacy testing if you plan to make performance claims
If your supplier has conducted any additional testing—even if not required—request those results. More data gives your safety assessor better insight into the product's safety profile.
What to Do When Suppliers Won't Provide Documentation
Some suppliers resist sharing detailed formulation information, viewing it as proprietary. Here's how to navigate this challenge:
If your supplier still refuses, consider these alternatives. Some suppliers will work directly with your safety assessor, providing documentation confidentially to them while keeping it from you. This protects their formulation secrecy while enabling your compliance. You might accept a less detailed CPSR based on limited information, though this may restrict what claims you can make or how you can market the product.
As a last resort, laboratory analysis can reverse-engineer the formulation, though this adds significant cost (€1,000-3,000) and time (6-8 weeks) to your project.
For an in-depth understanding of documentation requirements, review our complete CPSR preparation guide.
CPNP Registration for White Label Brands: Your Final Compliance Step
After obtaining your CPSR, you must notify your product through the Cosmetic Product Notification Portal before placing it on the EU market. This notification explicitly links your product, your company as Responsible Person, and your CPSR.
The CPNP Notification Process
CPNP notification is mandatory for every cosmetic product entering the EU market. The system serves multiple purposes: it allows competent authorities to monitor cosmetic products, provides critical information for poison control centers in medical emergencies, and creates a searchable database for market surveillance.
Your Responsible Person submits the notification, which includes product identity (name, category, intended function), complete formulation, label images showing all consumer-facing text, your contact information as the Responsible Person, product safety information referencing your CPSR, and frame formulation ranges if you have color variations.
The CPNP system is free to use—there are no registration fees. However, the notification must be completed before your product's market launch. Allow yourself 10-14 business days before your planned launch date, as technical issues occasionally arise during submission.
Common CPNP Submission Issues for White Label Brands
White label brands frequently encounter specific challenges during CPNP notification. The most common issue is formulation mismatches—the formula you submit must exactly match what's in your CPSR. Even minor discrepancies in ingredient order or concentration ranges will cause problems.
Label compliance is another frequent stumbling block. Your label images must show all mandatory information clearly legible, including your Responsible Person's name and address exactly as shown in your CPSR, complete ingredient lists in descending order of weight, and any required warnings or usage instructions.
Product categorization confusion also trips up many brands. The CPNP uses specific product categories and codes. Choose the category that most accurately reflects your product's intended use, as this affects which ingredients are permitted and what concentration limits apply.
For detailed guidance on navigating the notification process, see our CPNP registration step-by-step guide.
Managing CPNP for Multiple Markets
If you plan to sell across multiple EU countries, a single CPNP notification covers all EU member states. You don't need separate notifications for France, Germany, Italy, etc. The portal automatically distributes your notification to all national competent authorities.
However, if you're also targeting the UK market (post-Brexit), you need a separate notification through the UK's SCPN (Submit Cosmetic Product Notifications) portal. The UK system mirrors CPNP functionality but maintains independence from the EU database.
Common Compliance Mistakes to Avoid When Launching White Label Cosmetics
After working with hundreds of white label cosmetic brands, regulatory consultants consistently see the same compliance mistakes. Avoiding these pitfalls saves time, money, and potential legal issues.
Assuming Supplier Compliance Transfers to You
The most dangerous assumption is believing that because your supplier is compliant, you automatically are too. Your supplier's compliance protects them—not you. When you rebrand and sell the product, you become liable. Authorities will hold you accountable regardless of what your supplier told you about their compliance status.
Always verify compliance for yourself. Request documentation, engage your own safety assessor, and maintain your own PIF. Don't rely on supplier promises or certificates that you cannot verify.
Underestimating Lead Times
Many brands discover compliance requirements too late in their launch timeline. The complete process from engaging a safety assessor to CPNP notification takes 8-12 weeks for straightforward products. Complex formulations, missing documentation, or products requiring additional testing can extend this to 4-6 months.
Start your compliance process as soon as you select your white label products—not when your packaging arrives or your website launches. Regulatory work happens on the critical path of your product launch.
Incomplete Documentation from Suppliers
Discovering that your supplier cannot or will not provide necessary documentation after you've committed to products creates serious problems. Before placing significant orders or finalizing branding, confirm your supplier can supply complete formulation data, stability and safety testing results, GMP certificates, and detailed manufacturing specifications.
Build documentation requirements into your supplier agreements. Specify that the supplier must provide all information necessary for EU CPSR preparation as a condition of your business relationship.
Making Unsupported Claims
Your CPSR must support any efficacy or performance claims you make about your product. If your supplier makes modest claims but you want to make bolder statements—"reduces wrinkles by 40%" or "clinically proven results"—you need substantiation data in your PIF.
Claims substantiation is part of your Responsible Person's obligations. Authorities can request proof at any time. For guidance on compliant claims, review EU cosmetic claims regulations.
Ignoring Ingredient Compliance
Just because your supplier uses an ingredient doesn't mean it's permitted in the EU or at your intended concentration. Restricted substances, banned ingredients, and concentration limits differ between markets. The US FDA allows ingredients that the EU bans, and vice versa.
Your safety assessor evaluates ingredient compliance, but you should conduct a preliminary check using Annexes II (prohibited substances), III (restricted substances), and IV (allowed colorants) of the Cosmetics Regulation. Discovering non-compliant ingredients late in the process means reformulation—expensive and time-consuming.
For detailed ingredient compliance information, consult our ingredient compliance guide.
Cost and Timeline Expectations for White Label CPSR Preparation
Understanding realistic costs and timelines helps you budget appropriately and plan your launch schedule. Prices vary considerably based on product complexity, assessor experience, and whether you need additional services like Responsible Person representation.
CPSR Preparation Costs
For straightforward white label products—simple moisturizers, cleansers, or shampoos with well-established ingredients—expect to pay €500-800 per product for CPSR preparation. This assumes you provide complete formulation and testing documentation, and the product doesn't require novel ingredient evaluation.
Complex products command higher fees. Sunscreens, products with multiple actives, novel ingredients, or leave-on treatments with sophisticated delivery systems typically cost €1,200-2,000 per CPSR. The additional cost reflects the more extensive toxicological evaluation required.
Some assessors offer volume discounts if you're launching multiple products simultaneously. Expect 10-20% discounts when commissioning CPSRs for 5 or more products from the same supplier with similar complexity.
| Service Component | Typical Cost Range | Notes |
|---|---|---|
| CPSR Preparation (Simple Product) | €500-800 | Basic formulations, complete documentation provided |
| CPSR Preparation (Complex Product) | €1,200-2,000 | Novel ingredients, sunscreens, or sophisticated actives |
| CPSR Transfer/Validation | €200-500 | When supplier offers pre-assessed products |
| Responsible Person Services (Annual) | €300-800 per product | Includes PIF maintenance and regulatory monitoring |
| CPNP Notification | €50-150 per product | Portal submission service (portal itself is free) |
| Additional Testing (if needed) | €500-3,000 | Stability, challenge testing, or chemical analysis |
Hidden Costs to Budget For
Beyond direct CPSR fees, consider these additional expenses. If your supplier's documentation is incomplete, you may need supplementary testing. Stability testing costs €800-1,500 per product and takes 3-6 months. Challenge testing (preservative efficacy) runs €600-1,200 and requires 4-6 weeks. Chemical analysis to verify formulation or identify unknown ingredients costs €1,000-3,000.
If you need multiple language versions of your CPSR for different EU markets, translation services add €150-300 per language. Product liability insurance specifically covering EU markets typically costs €500-2,000 annually depending on your product category and sales volume.
Realistic Timeline Planning
For a straightforward white label product where you have complete documentation, the typical timeline looks like this:
- Week 1-2: Gather documentation from supplier, engage safety assessor, provide initial information package
- Week 3-6: Safety assessor reviews documentation, conducts toxicological evaluation, prepares CPSR draft
- Week 7: Review CPSR, address any questions or required clarifications
- Week 8: Receive final signed CPSR, prepare CPNP submission
- Week 9-10: Complete CPNP notification, receive confirmation
This 10-week timeline assumes no complications. Add buffer time for potential issues like missing test data, ingredient compliance questions, or formulation concerns that require supplier consultation.
Complex products or situations requiring additional testing can extend timelines significantly. Stability testing alone requires 12-24 weeks depending on claimed shelf life. Challenge testing needs 28-42 days. If you discover ingredient compliance issues requiring reformulation, add 8-12 weeks for supplier reformulation and revalidation.
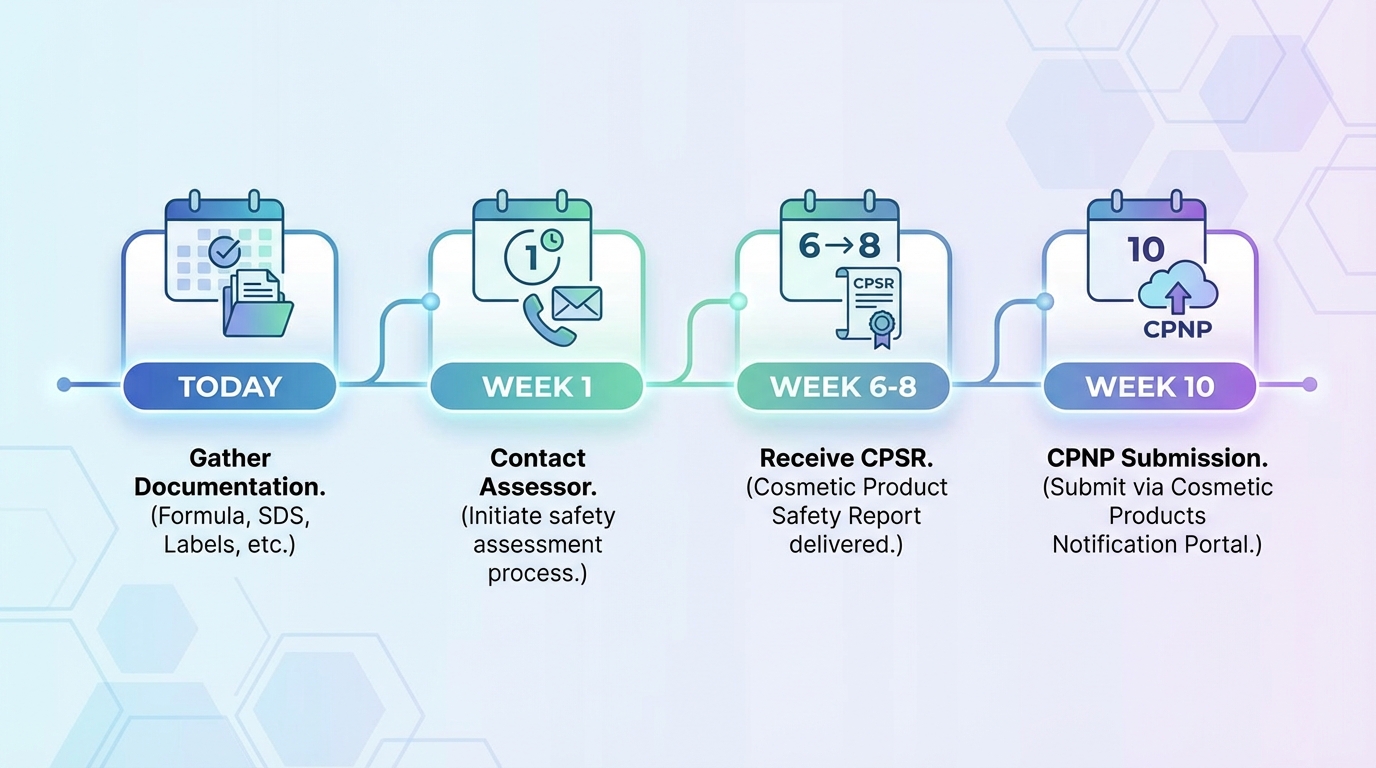
Real-World Case Study: White Label Skincare Brand Launch
To illustrate how CPSR requirements play out in practice, consider a typical scenario that regulatory consultants encounter regularly with white label brands.
A UK-based entrepreneur wants to launch a premium skincare line targeting the European market. She identifies a reputable Italian white label manufacturer offering five products: vitamin C serum, hyaluronic acid moisturizer, retinol night cream, cleanser, and eye cream.
The manufacturer assures her that all products are "fully compliant" and "ready for the EU market." The products arrive beautifully packaged with the manufacturer's branding, which she plans to remove and replace with her own labels.
When she begins investigating EU requirements, she learns about CPSR and CPNP obligations. She contacts the manufacturer requesting their CPSR documents. The manufacturer explains they have CPSRs for all products—but those reports list the manufacturer as the Responsible Person.
The manufacturer offers two options:
- Introduce her to their consultant (€650/product for new CPSRs)
- Share documentation under NDA with her chosen consultant
Strategic Choice
She chooses an independent regulatory consultant who offers a package deal:
- CPSR preparation for all 5 products (€3,250 total)
- EU Responsible Person services (€2,000 annually)
- CPNP notification (€400 total)
- Ongoing monitoring and PIF maintenance
Process & Timeline
The process takes 9 weeks. The consultant identifies one minor issue—the retinol concentration requires specific label warnings not on the original labels. She adjusts her artwork accordingly.
Her total first-year compliance investment: €5,650 for initial setup plus €2,000 annually for ongoing RP services.
She launches successfully in France, Germany, and the Netherlands. Within the first year, a French competent authority conducts a market surveillance check, requesting her PIF. Her regulatory consultant responds within 48 hours with complete documentation, and the authority confirms compliance.
Key Point:This case illustrates several best practices: engaging regulatory support early, budgeting for compliance costs, and understanding that "white label" does not mean "compliance-free."
Implementation Timeline and Next Steps
You now understand why white label cosmetics almost always require a new CPSR and how to navigate the compliance process. Here's your actionable implementation roadmap organized by timeframe.
Today: Immediate Actions
This Week: Planning & Coordination
- Obtain Supplier QuotesCompare supplier's regulatory support pricing vs. independent consultants.
- Research ConsultantsIdentify 3 qualified safety assessors. Request quotes and compare service packages.
- Review FormulationsConduct preliminary ingredient checks against EU annexes to spot red flags early.
- Map Your TimelineWork backward from launch date. Build in buffer time for unexpected delays.
This Month: Engage Professional Support
Engage Partner
Select safety assessor/RP and formalize engagement. Clarify deliverables.
Gather Documentation
Compile formulation data, test results, and supplier certifications.
Establish RP
Decide on own entity vs. third-party service. Sign mandates.
Finalize Labels
Create artwork with all mandatory info. Have consultant review before printing.
Ongoing: Maintenance & Monitoring
Frequently Asked Questions
Sources & References
- 1. European Commission — Regulation (EC) No 1223/2009 on Cosmetic Products — November 30, 2009
- 2. Zignify — White Label Cosmetic Products: Trends, Compliance & Launch Guide — September 25, 2025
- 3. Certified Cosmetics — How to Get a CPSR for Cosmetic Product (Step-by-Step) — 2025
- 4. Brilitas EU — CPSR for Whitelabel and Private Label Products: Strategic Solutions — December 18, 2025
- 5. Aurora Cosmetic — Mistakes to Avoid in Private Label Cosmetics Launch — January 12, 2026
- 6. Biorius — EU Cosmetics Regulation Comprehensive Guide — 2025
- 7. EcoMundo — Understanding the Role of a Responsible Person in Cosmetics — 2025
- 8. Obelis — EU Cosmetic Compliance: Guide to Entering the EU Market — May 22, 2025
- 9. MSDS Europe — The Responsibilities of the Responsible Person Regarding Cosmetics — 2025
- 10. Phoenix Cosmetic Safety — Product Information File for EU Cosmetics Compliance 2026 Edition — December 23, 2025